
The ABC of the battery industry
The battery value chain industry is growing rapidly in Finland, and the country is becoming an international leader in the battery and electrification sector. Finland’s logistical opportunities, responsible production and energy expertise are huge assets that are attracting investors in the sector from all over the world.
The electrification of society and the solutions offered by the battery value chain industry will play a major role in the achievement of climate targets. The Finnish Ministry of Economic Affairs and Employment has drawn up a national battery strategy to attract investments of billions of euros to the country and to create numerous new battery value chain companies and thousands of jobs. Training programmes and modules are currently being developed at universities across the country to meet the demand for skilled experts.
Several battery value chain projects have been launched in Finland, many of which have already reached or are close to reaching the investment stage. In Kokkola, for example, preparations are underway to produce battery grade lithium hydroxide refinery, while five lithium mines are planned to be built in Kaustinen. In addition, the GigaVaasa area, under construction in Vaasa, is set to become a synergistic home for several companies in the sector.
Finland’s strict legislation and attitude to environmental responsibility guarantee that batteries are manufactured sustainably. Efforts are constantly being made, for example, to find the most optimal solutions for climate protection. Battery value chain processes require a huge amount of energy, and in Finland they can largely be carried out using clean wind power. This renewable energy is available in record quantities and, in a sparsely populated country like Finland, not all the wind power capacity available has been harnessed yet. There are also other excellent energy solutions available: for example, the waste heat from GigaVaasa’s battery factories will be used in the district heating network, and the operators will even be compensated for it.
Battery value chain
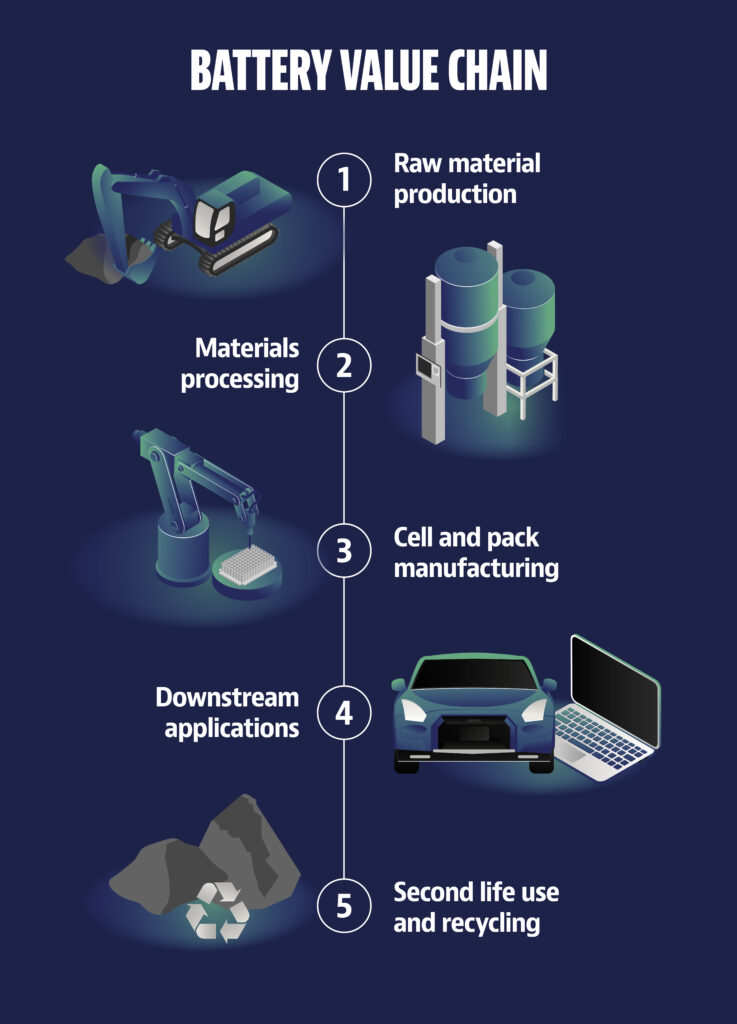
1. Raw material production
Rock is quarried from mines and the metals needed for batteries are extracted from the rock. The metals, elements and chemical compounds needed in the battery industry are nickel, manganese, cobalt, lithium, iron, phosphate and graphite. Nickel, cobalt and lithium can be extracted from Finnish bedrock.
2. Materials processing
The raw-mined metals from the mine are processed into precursors, from which cathode material is processed. Cathodes and anodes are mixtures of different metals, a black powder that is packed into large bags and shipped to cell factories.
3. Cell and pack manufacturing
In battery cell factories, cathodes and anodes are packed into cells separated by a layer of film. Finally, the cells are packaged into finished batteries, which can be very different designs depending on the final product. Battery cell factories are the most important actors in the battery value chain in terms of employment and investment.
4. Downstream applications
Batteries are needed for a wide range of applications, from individual cells powering, for example, torches, to rechargeable batteries for electronic devices, all the way to batteries for electric vehicles and energy reserves in power plants.
5. Second life use and recycling
The aim is closed-loop recycling and reuse of the metals from spent batteries. More and more efficient recycling solutions are constantly being developed.

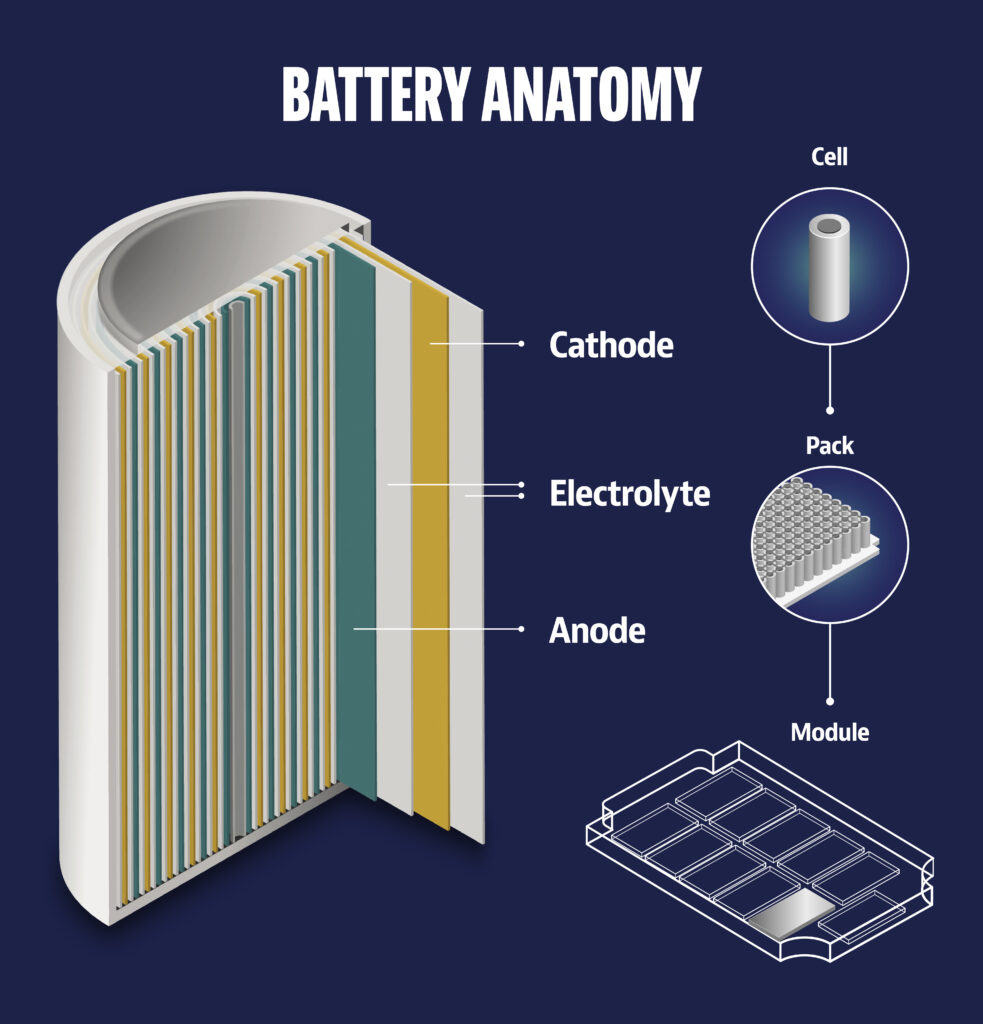
Battery anatomy
How it works?
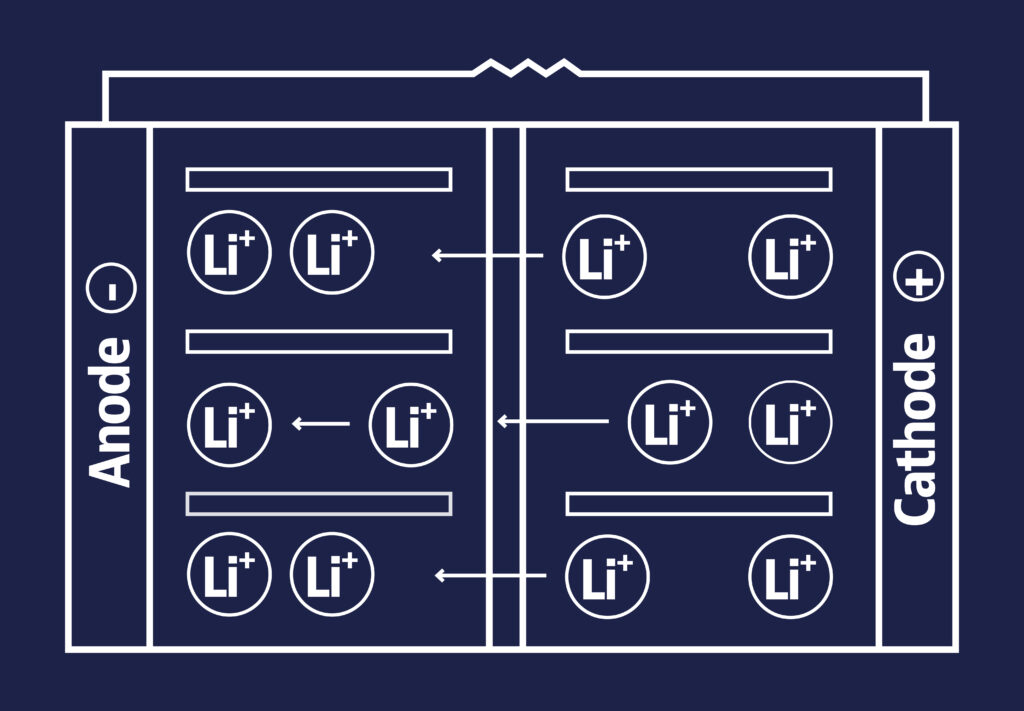
Lithium ions move from the negative electrode to the positive electrode during discharge and back when charging.
Cathode
A cathode is and electrode where reduction reactions occur, in which atoms gain electrons. Nickel, Manganese and cobalt are needed for the cathodes of NMC batteries, while lithium, iron and phosphates are needed fo LFP batteries. Lithium, iron and phosphates also work best in the cathodes of battery storage facilities.
Electrolyte
The electrolyte is the medium that transports positively charged ions between the cathode and anode terminals. Depending on the type of battery, electrolyte can be a liquid or paste-like substance. It consists of soluble salts, acids or other bases.
Anode
An anode is an electrode where oxidation reactions occur, involving atoms giving up electrons. The negatively charged free electrons then flow out of the negative terminal of the battery to produce an electrical current.
The anode material for batteries is graphite, a crystalline form of carbon. It can be extracted from mines or produced in a bio-based way by processing lignin extracted from wood. To remove impurities from graphite, it must be heated to 3,000 degrees Celsius during the downstream processing stage.






